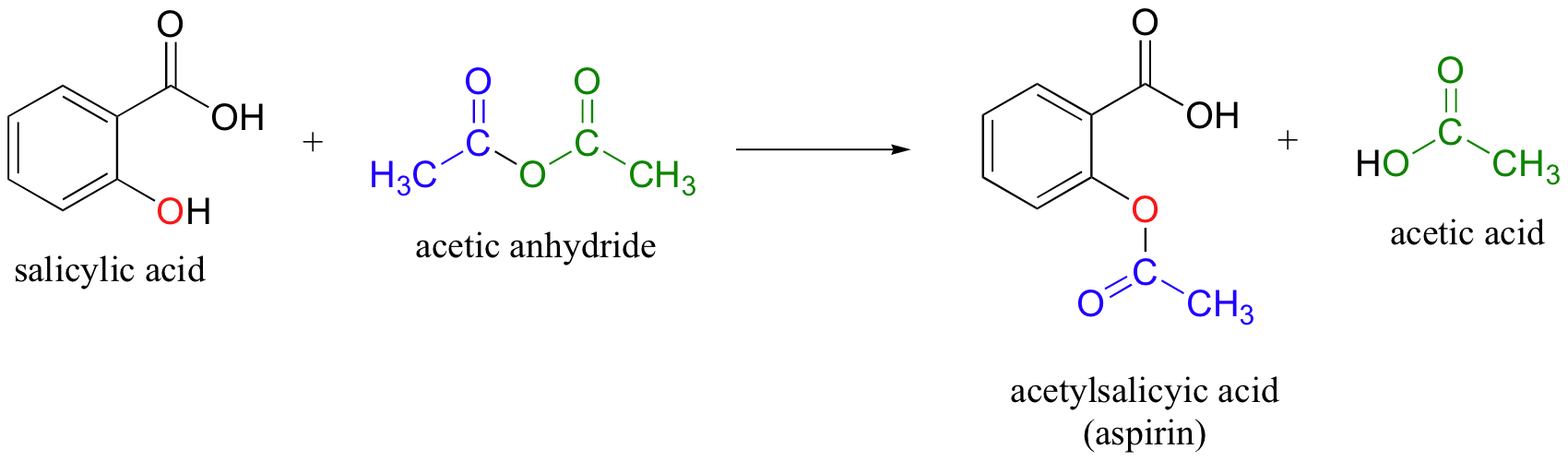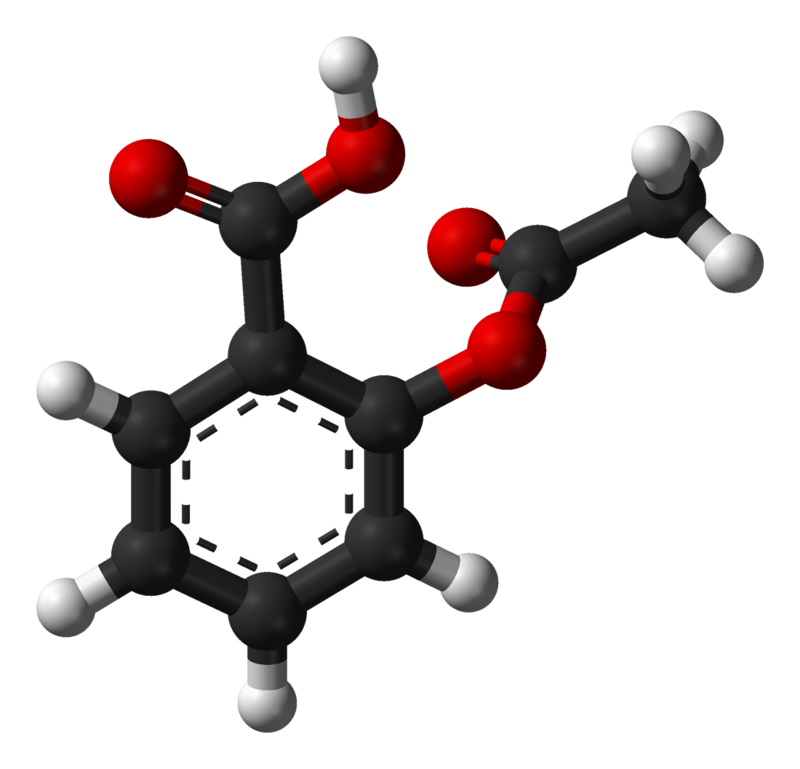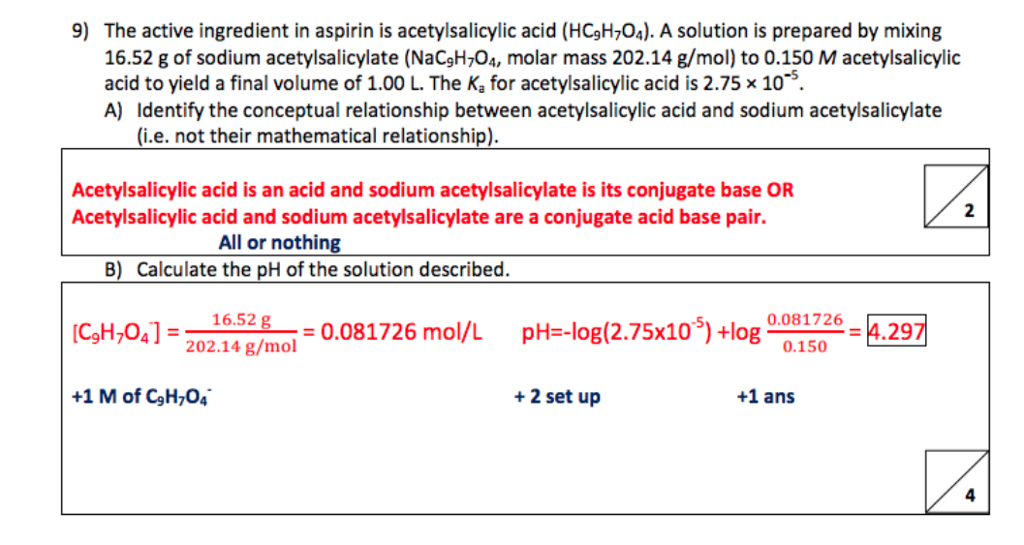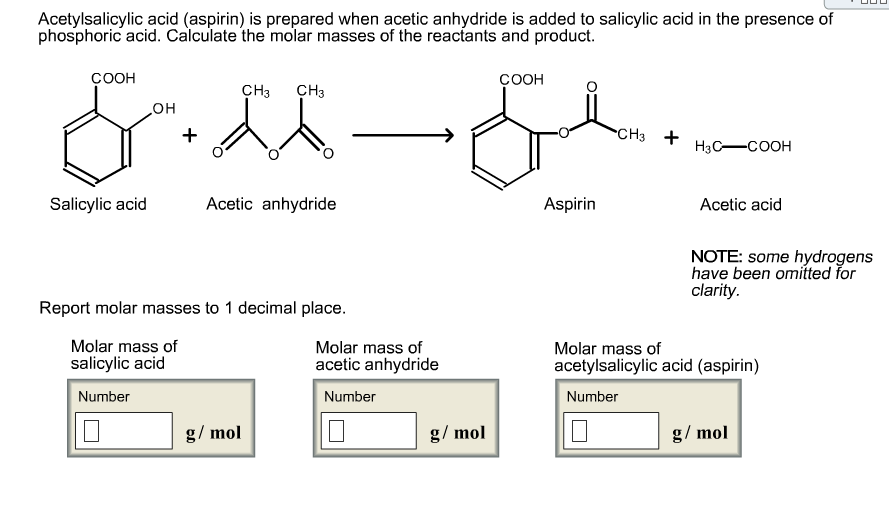
SOLVED: If one mole of salicylic acid (molar mass=138.12 g/mol) reacts with 1 mole of acetic anhydride (molar mass=102.09 g/mol) , it will yield 1 mole of aspirin (or acetylsalicylic acid; molar

Sketch the molecular structure of acetylsalicylic acid and calculate its molar mass. | Homework.Study.com

SOLVED: The molar mass of salicylic acid (CzH6O3) is 138 g/mole: The molar mass of [acetylsalicylic acid (CgH,O4) is 180 g/mole. Calculate the theoretical yield of acetylsalicylic acid (aspirin) based on thc

SOLVED: (T) Aspirin (CoHsO+: molar mass = 180.157 glmol) is produced from the reaction of salicylic acid (C-HsOa, molar mass = 138.1207 g/mol) and acetic anhydride (CAHsO3 molar mass 102.0887 g/mol): CzHsO3 +

SOLVED: 3-The chemical formula of aspirin is C9H804. What is the mass of 0.40 mol of aspirin? A) 72 g B) 45 g C) 160 g D) 10.8 g
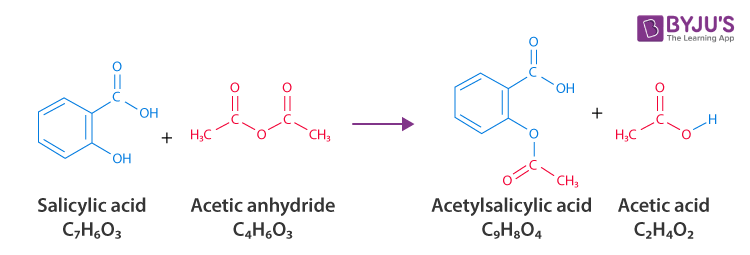
Acetylsalicylic acid (Aspirin ) - C9H8O4 - Formula, Structure, Properties, Preparation, Uses, Health risk and FAQs of Aspirin/ Acetylsalicylic ((C9H8O4)

SOLVED: The molar mass of salicylic acid (CzH6O3) is 138 g/mole: The molar mass of [acetylsalicylic acid (CgH,O4) is 180 g/mole. Calculate the theoretical yield of acetylsalicylic acid (aspirin) based on thc

Acetyl salicylic acid (mol. wt. = 180) called aspirin is a pain killer with pKa = 6 . It two tablets each of 0.09 gm mass containing aspirin are dissolved in 100 mL solution. Its pH will be:
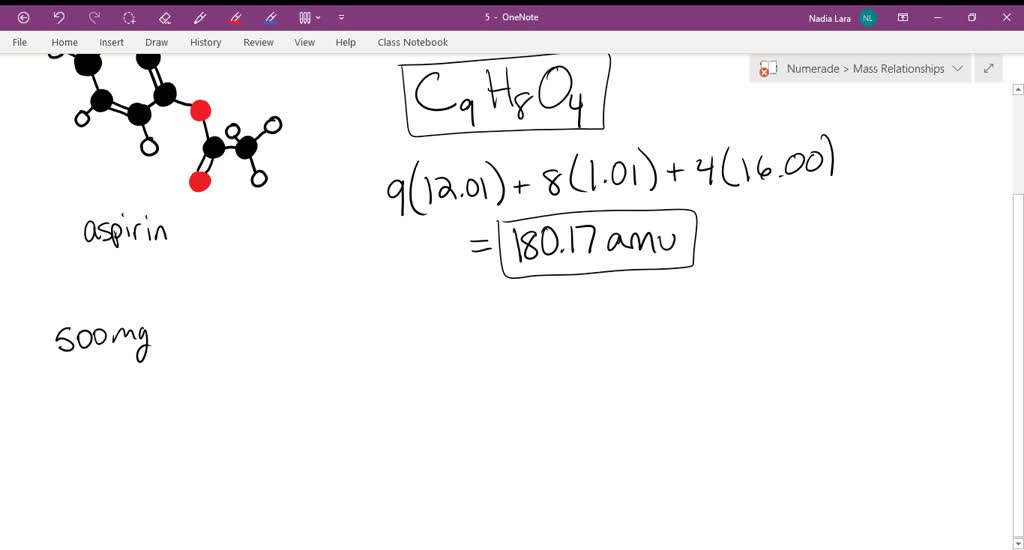
SOLVED:Aspirin can be represented by the adjacent ball-and-stick molecular model. Give the formula for aspirin, and calculate its molecular mass (red =O, gray =C, ivory =H ). How many moles of aspirin