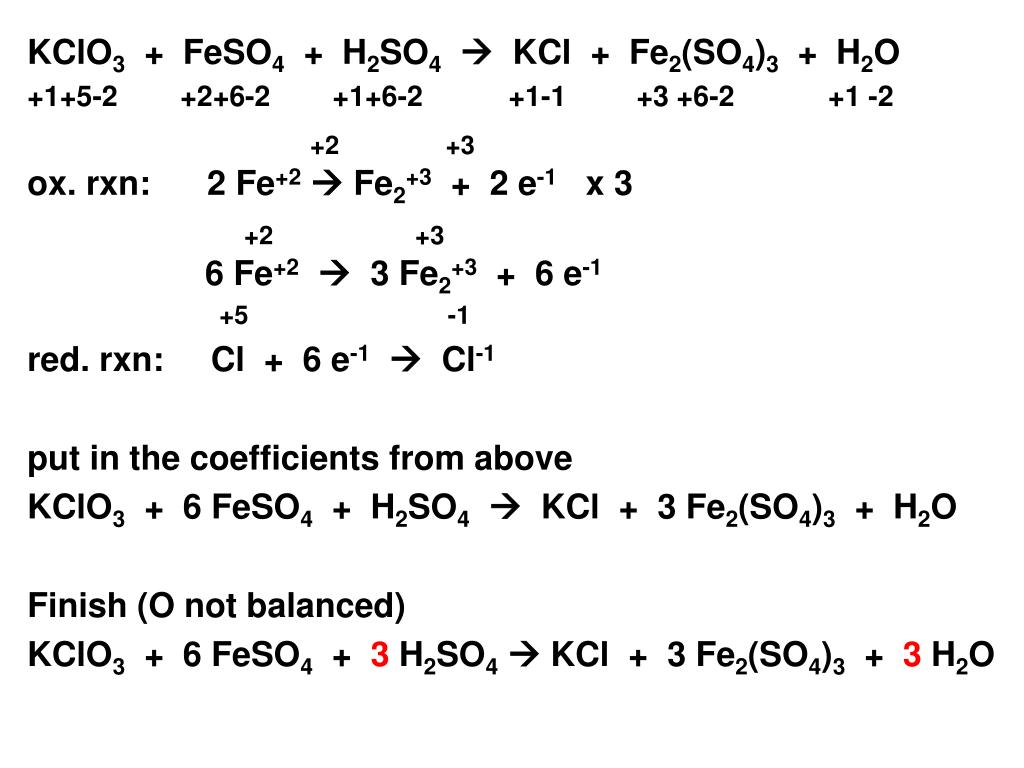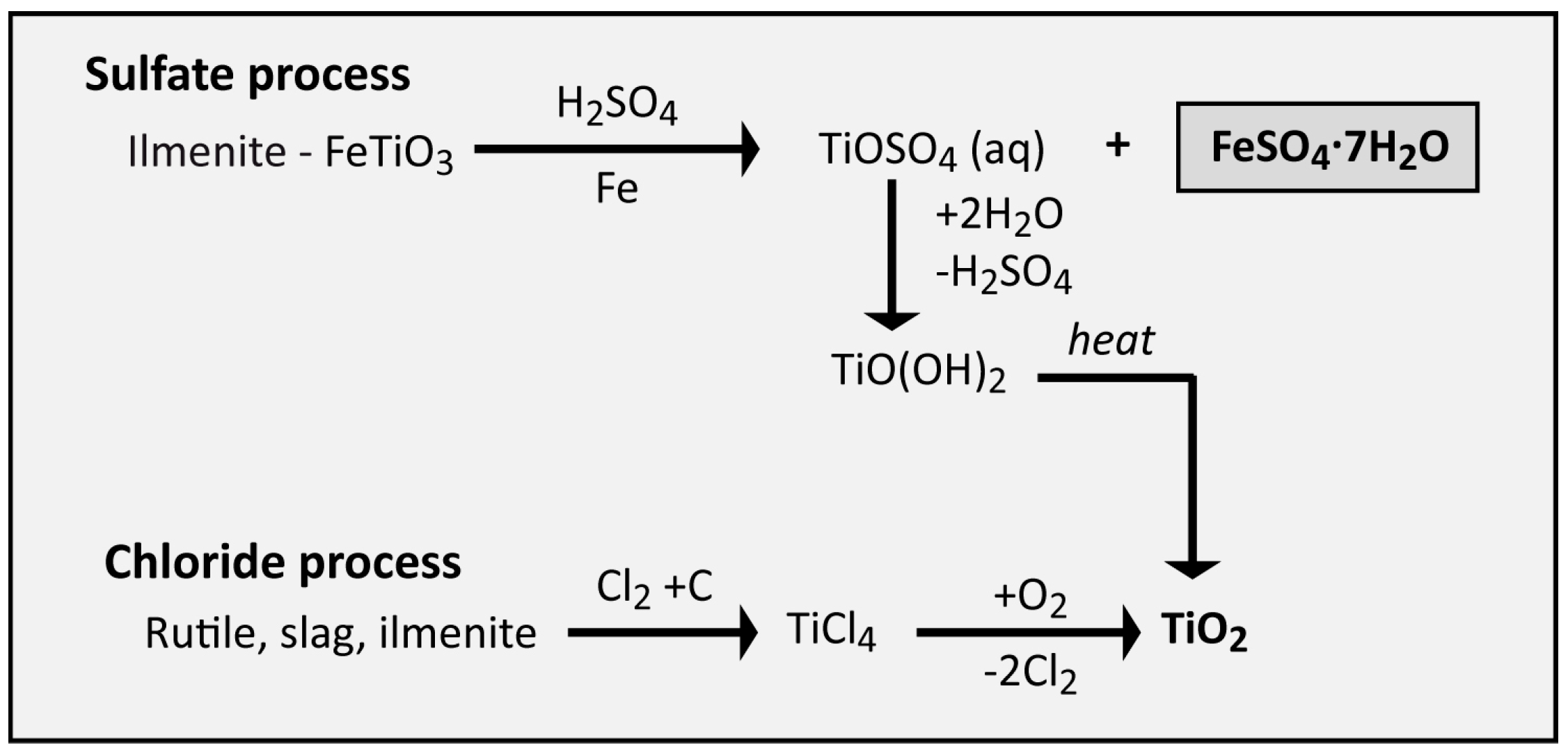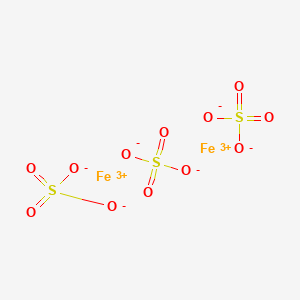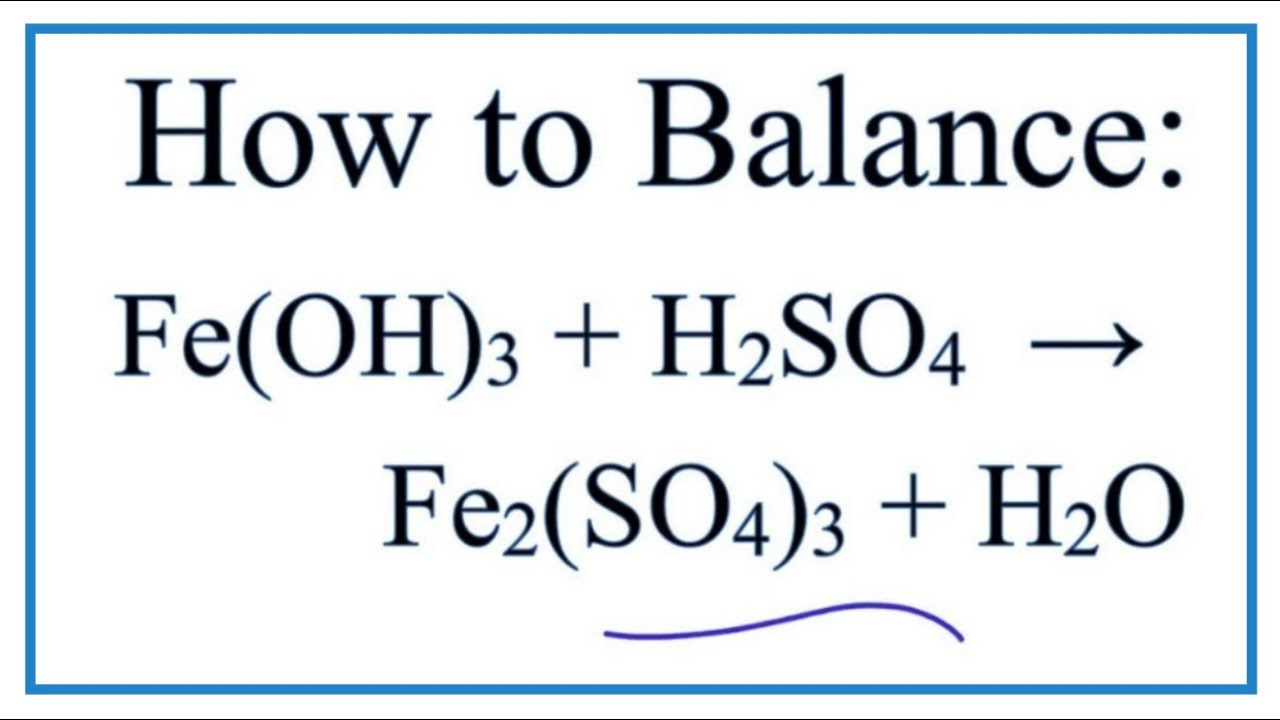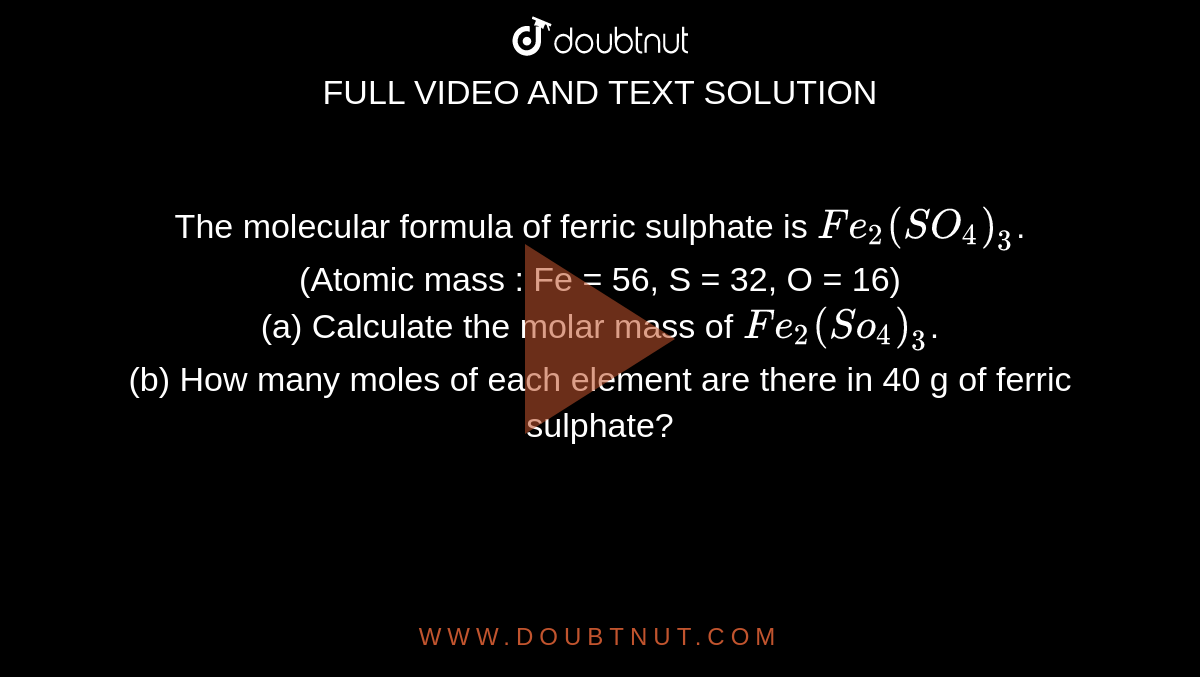
The molecular formula of ferric sulphate is Fe2(SO4)3. (Atomic mass : Fe = 56, S = 32, O = 16) (a) Calculate the molar mass of Fe2(So4)3. (b) How many moles of

Difference Between Ferrous Gluconate and Ferrous Sulfate | Compare the Difference Between Similar Terms

Question Video: Identifying Iron Oxide Produced from the Reaction of Unknown Salt with an Alkali Solution | Nagwa

What is the colour of FeSO4 . 7H2O crystals ? How does this colour change upon heating ? Give balanced chemical equation for the changes.

Iron(III) Sulfate as Terminal Oxidant in the Synthesis of Methyl Ketones via Wacker Oxidation | The Journal of Organic Chemistry

Fe + CuSO4 = FeSO4 + Cu,find oxidation, reduction, oxidising agent and reducing agent. - Brainly.in
In order to oxidise a mixture of one mole of each of FeC2O4, Fe2(C2O4)3, FeSO4 and Fe2(SO4)3 - Sarthaks eConnect | Largest Online Education Community

The Oxidation of Fe(II) in Acidic Sulfate Solutions with Air at Elevated Pressures. Part 1. Kinetics above 1 M H2SO4 | Industrial & Engineering Chemistry Research
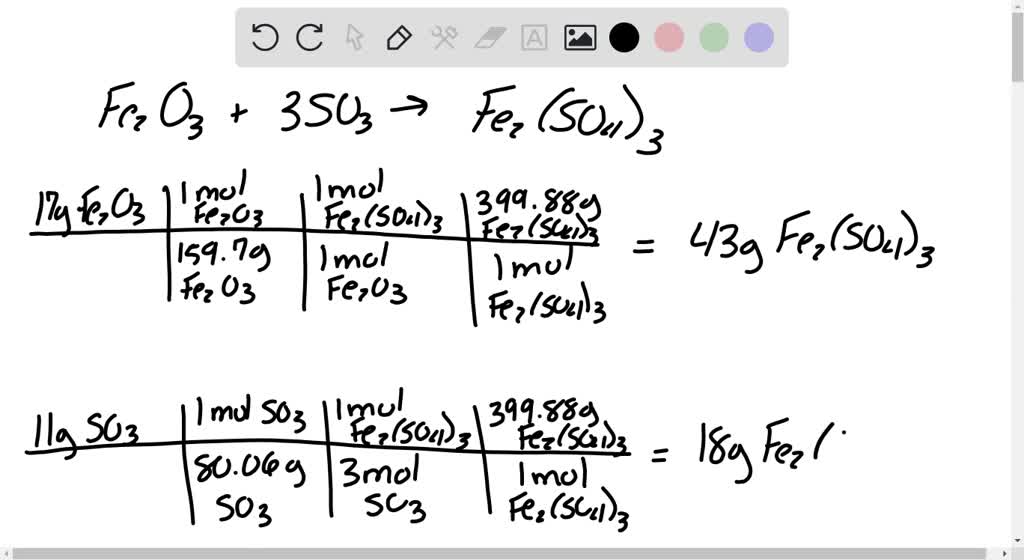
SOLVED: Fe2O3 + 3SO3 -> Fe2(SO4)31) you have 17g of iron(III)oxide. how many grams of iron(III)sulfate are produced? show unit analysis! 2) you have 11g of sulfur trioxide. how many grams of